Difference between revisions of "Eichhornia crassipes"
in A. L. P. P. de Candolle and C. de Candolle, Monogr. Phan. 4: 527. 1883.
FNA>Volume Importer |
FNA>Volume Importer |
||
| Line 14: | Line 14: | ||
|name=Eichhornia speciosa | |name=Eichhornia speciosa | ||
|authority=Kunth | |authority=Kunth | ||
| − | }}{{Treatment/ID/Synonym | + | }} {{Treatment/ID/Synonym |
|name=Piaropus crassipes | |name=Piaropus crassipes | ||
|authority=(Martius) Rafinesque | |authority=(Martius) Rafinesque | ||
| Line 33: | Line 33: | ||
|distribution=Ala.;Ariz.;Calif.;Fla.;Ga.;La.;Miss.;N.C.;S.C.;Tex.;Va.;worldwide;tropics and subtropics. | |distribution=Ala.;Ariz.;Calif.;Fla.;Ga.;La.;Miss.;N.C.;S.C.;Tex.;Va.;worldwide;tropics and subtropics. | ||
|discussion=<p>This is probably the most aggressive aquatic weed ever known in the tropics (T. D. Center and N. R. Spencer 1981; R. E. Fitzsimons and R. H. Vallejos 1986; W. T. Penfound and T. T. Earle 1948; H. J. Webber 1897) and has been documented to cause extensive physiological changes in the aquatic environment (G. R. Ultsch 1973). It is on the USDA/APHIS noxious weeds list and the Florida Department of Natural Resources prohibited list (D. C. Schmitz 1990). Extensive research has gone into pesticide and biological control (R. G. Baer and P. C. Quimby Jr. 1981; T. D. Center and T. K. Van 1989; J. C. Joyce and W. T. Haller 1985; D. C. Schmitz 1990), resulting in its decline in Florida. Plants were probably first introduced into the U.S. at the Louisiana exposition in 1884 (W. T. Penfound and T. T. Earle 1948), and the species is now known in all states where freezing temperatures are minimal. Research results from the 1970s suggest that the species may have an economic importance by removing toxic substances from water (F. Chigbo et al. 1982).</p><!-- | |discussion=<p>This is probably the most aggressive aquatic weed ever known in the tropics (T. D. Center and N. R. Spencer 1981; R. E. Fitzsimons and R. H. Vallejos 1986; W. T. Penfound and T. T. Earle 1948; H. J. Webber 1897) and has been documented to cause extensive physiological changes in the aquatic environment (G. R. Ultsch 1973). It is on the USDA/APHIS noxious weeds list and the Florida Department of Natural Resources prohibited list (D. C. Schmitz 1990). Extensive research has gone into pesticide and biological control (R. G. Baer and P. C. Quimby Jr. 1981; T. D. Center and T. K. Van 1989; J. C. Joyce and W. T. Haller 1985; D. C. Schmitz 1990), resulting in its decline in Florida. Plants were probably first introduced into the U.S. at the Louisiana exposition in 1884 (W. T. Penfound and T. T. Earle 1948), and the species is now known in all states where freezing temperatures are minimal. Research results from the 1970s suggest that the species may have an economic importance by removing toxic substances from water (F. Chigbo et al. 1982).</p><!-- | ||
| − | --><p>The inflated petiole has long been a characteristic used to separate Eichhornia crassipes from other species of the genus. Yet on older plants under crowded conditions, the petiole shows little or no swelling (W. T. Penfound and T. T. Earle 1948). This has resulted in several misidentifications of specimens as E. azurea.</p><!-- | + | --><p>The inflated petiole has long been a characteristic used to separate <i>Eichhornia crassipes</i> from other species of the genus. Yet on older plants under crowded conditions, the petiole shows little or no swelling (W. T. Penfound and T. T. Earle 1948). This has resulted in several misidentifications of specimens as <i>E. azurea</i>.</p><!-- |
--><p>Inflorescences typically elongate overnight, and all flowers open the same day (W. T. Penfound and T. T. Earle 1948). However, some robust specimens have inflorescences in which the proximal flowers open the first day and the distal 2–4 flowers open on the subsequent day. Individual flowers open shortly after dawn and wilt by nightfall. By the next morning, the flowering stem has bent such that the developing fruits are typically submersed, allowing seed development to occur under water (W. T. Penfound and T. T. Earle 1948).</p><!-- | --><p>Inflorescences typically elongate overnight, and all flowers open the same day (W. T. Penfound and T. T. Earle 1948). However, some robust specimens have inflorescences in which the proximal flowers open the first day and the distal 2–4 flowers open on the subsequent day. Individual flowers open shortly after dawn and wilt by nightfall. By the next morning, the flowering stem has bent such that the developing fruits are typically submersed, allowing seed development to occur under water (W. T. Penfound and T. T. Earle 1948).</p><!-- | ||
--><p>S. C. H. Barrett (1977, 1979, 1980, 1980b, 1988, 1989) has studied the reproductive biology in depth. The water hyacinth is a tristylous species; however, the only naturally occuring populations with all three morphs are in northeastern Brazil. This led S. C. H. Barrett and I. W. Forno (1982) to conclude that Eichornia crassipes is native to Amazonia. All other studied populations in the world have only one or two of the floral morphs. In some populations, a breakdown in the tristylous condition was observed whereby the stigma was found to be adjacent to anthers in some flowers (semihomostylous condition), resulting in some seed production (S. C. H. Barrett 1979). In general, seed production in temperate populations was found to be half that of tropical populations, mainly due to differences in levels of insect visitation (S. C. H. Barrett 1980b).</p> | --><p>S. C. H. Barrett (1977, 1979, 1980, 1980b, 1988, 1989) has studied the reproductive biology in depth. The water hyacinth is a tristylous species; however, the only naturally occuring populations with all three morphs are in northeastern Brazil. This led S. C. H. Barrett and I. W. Forno (1982) to conclude that Eichornia crassipes is native to Amazonia. All other studied populations in the world have only one or two of the floral morphs. In some populations, a breakdown in the tristylous condition was observed whereby the stigma was found to be adjacent to anthers in some flowers (semihomostylous condition), resulting in some seed production (S. C. H. Barrett 1979). In general, seed production in temperate populations was found to be half that of tropical populations, mainly due to differences in levels of insect visitation (S. C. H. Barrett 1980b).</p> | ||
| Line 60: | Line 60: | ||
|publication year=1883 | |publication year=1883 | ||
|special status= | |special status= | ||
| − | |source xml=https://jpend@bitbucket.org/aafc-mbb/fna-data-curation.git/src/ | + | |source xml=https://jpend@bitbucket.org/aafc-mbb/fna-data-curation.git/src/8f726806613d60c220dc4493de13607dd3150896/coarse_grained_fna_xml/V26/V26_5.xml |
|genus=Eichhornia | |genus=Eichhornia | ||
|species=Eichhornia crassipes | |species=Eichhornia crassipes | ||
Revision as of 17:45, 18 September 2019
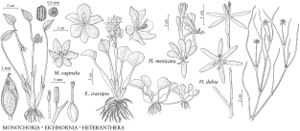
Plants perennial, typically free-floating. Vegetative stems condensed, except when branching. Flowering stems erect, bending over after flowering, to 25 cm, distal internode less than 4 cm. Sessile leaves in basal rosette. Petiolate leaves floating or emersed; stipule 2.5–14 cm, apex truncate; petiole at least slightly swollen, 3.5–33 cm; blade ovate to round, 2.5–11 × 3.5–9.5 cm. Spikes 4–15-flowered; spathes obovate, 4–11 cm; peduncle 5–12.5 cm, glabrous. Flowers opening individually within 2 hours after sunrise, wilting by night; perianth blue or mauve-blue, limb lobes obovate, 16–37 mm, margins entire, central distal lobe with dark blotch in center and yellow spot within blotch; proximal stamens 20–35 mm, distal 14–19 mm; anthers 1.7–2.1 mm; style 3-lobed. Seeds 11–14-winged, 1.1–2.1 × 0.6–0.9 mm. 2n = 32.
Phenology: Flowering early spring–late fall.
Habitat: Ponds, ditches, canals, calm waters of rivers
Elevation: 0–300 m
Distribution
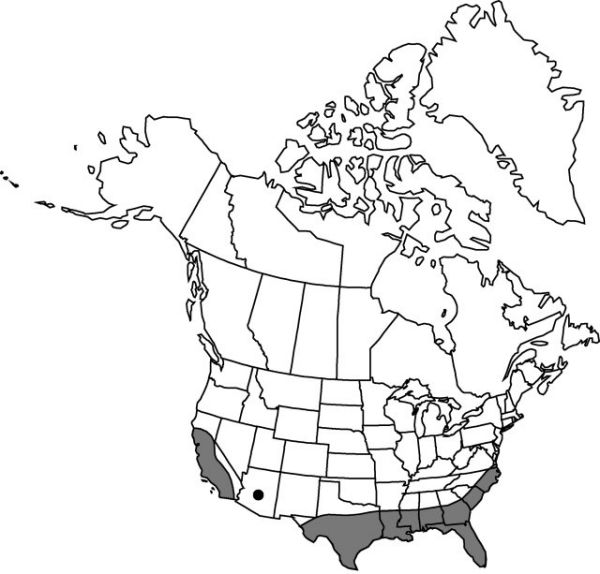
Ala., Ariz., Calif., Fla., Ga., La., Miss., N.C., S.C., Tex., Va., worldwide, tropics and subtropics.
Discussion
This is probably the most aggressive aquatic weed ever known in the tropics (T. D. Center and N. R. Spencer 1981; R. E. Fitzsimons and R. H. Vallejos 1986; W. T. Penfound and T. T. Earle 1948; H. J. Webber 1897) and has been documented to cause extensive physiological changes in the aquatic environment (G. R. Ultsch 1973). It is on the USDA/APHIS noxious weeds list and the Florida Department of Natural Resources prohibited list (D. C. Schmitz 1990). Extensive research has gone into pesticide and biological control (R. G. Baer and P. C. Quimby Jr. 1981; T. D. Center and T. K. Van 1989; J. C. Joyce and W. T. Haller 1985; D. C. Schmitz 1990), resulting in its decline in Florida. Plants were probably first introduced into the U.S. at the Louisiana exposition in 1884 (W. T. Penfound and T. T. Earle 1948), and the species is now known in all states where freezing temperatures are minimal. Research results from the 1970s suggest that the species may have an economic importance by removing toxic substances from water (F. Chigbo et al. 1982).
The inflated petiole has long been a characteristic used to separate Eichhornia crassipes from other species of the genus. Yet on older plants under crowded conditions, the petiole shows little or no swelling (W. T. Penfound and T. T. Earle 1948). This has resulted in several misidentifications of specimens as E. azurea.
Inflorescences typically elongate overnight, and all flowers open the same day (W. T. Penfound and T. T. Earle 1948). However, some robust specimens have inflorescences in which the proximal flowers open the first day and the distal 2–4 flowers open on the subsequent day. Individual flowers open shortly after dawn and wilt by nightfall. By the next morning, the flowering stem has bent such that the developing fruits are typically submersed, allowing seed development to occur under water (W. T. Penfound and T. T. Earle 1948).
S. C. H. Barrett (1977, 1979, 1980, 1980b, 1988, 1989) has studied the reproductive biology in depth. The water hyacinth is a tristylous species; however, the only naturally occuring populations with all three morphs are in northeastern Brazil. This led S. C. H. Barrett and I. W. Forno (1982) to conclude that Eichornia crassipes is native to Amazonia. All other studied populations in the world have only one or two of the floral morphs. In some populations, a breakdown in the tristylous condition was observed whereby the stigma was found to be adjacent to anthers in some flowers (semihomostylous condition), resulting in some seed production (S. C. H. Barrett 1979). In general, seed production in temperate populations was found to be half that of tropical populations, mainly due to differences in levels of insect visitation (S. C. H. Barrett 1980b).
Selected References
None.